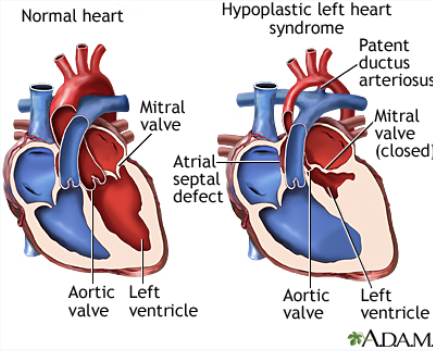
In this project, I designed an optimization along with three other biomedical engineers for a cardiovascular surgery
conducted on babies with a disorder known as hypoplastic left heart syndrome.
Background
If you've never learned about the anatomy of the heart, picture it as two chambers working in tandem. The left side, which
houses the left ventricle and left atrium, pumps oxygenated blood for the rest of the blood to use as energy. The right side,
which houses the right ventricle and right atrium, takes the blood that is devoid of oxygen and send it to lungs for oxygenation.
Hypoplastic left heart syndrome is a congenital disorder which causes a marked decrease in left ventricle volume; so much so that the
contribution from the compartment is considered negligible (note the picture on the right, left ventricle size is substantially reduced).
Without the left ventricle, oxygenated blood can't get to thetissues that need it, which means no nutrients and eventually, tissue death.
When the baby is in the womb this is fine, as nutrients are supplied through the mother. But, when the baby is born, the heart cannot
function in a self-sufficient manner. Left untreated, it could be fatal..
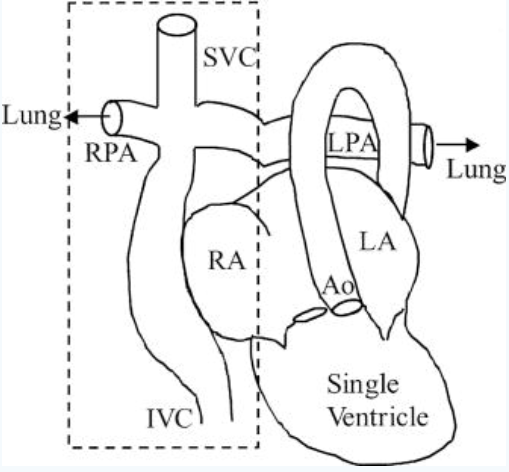
The condition is usually treated through a three-step surgery known blandly as staged palliation, which occurs over 1.5 years approximately
every 6 months. The survival rate for children at age 5 is about 70 percent and most of these children have normal growth and development.
Following the surgery, the two chamber heart is effectively converted into a single chamber heart. That single compartment now pumps
oxygenated blood from the lungs to the rest of the body. The vessels that brought deoxygenated blood to the heart, the superior and inferior
vena cava (SVC and IVC), are cut off from the heart and now connected to the pulmonary artery, which previously brought deoxygenated blood
from the heart to the lungs to be oxygenated. At a high level, our heart now pumps oxygenated blood to the rest of the body, it flows back
through the SVC and IVC into the pulmonary artery (without being pumped by the heart again like before the surgery) and into the lungs to be
oxygenated, then flows back into the heart as oxygenated blood. And the cycle continues.
Our main focus will be on the top left side on the picture to the left. The cross-like shape connection with the IVC and SVC is what is
called a total cavopulmonary connection, or TCPC for short.
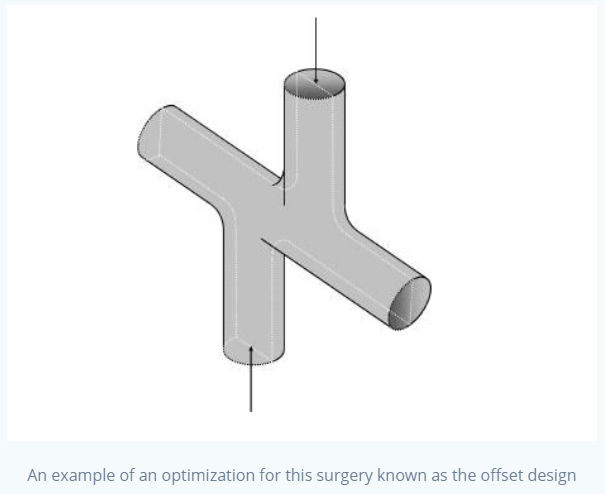
As you can imagine, while the surgery is generally successful, it doesn't come without complications.
Patients often have to be monitored annually after surgery and their ability to exercise, or just do
physically-taxing work, is significantly reduced. There have been a ton of optimizations that have been
introduced to help alleviate this.
Let's go back to that cross-like shape. This is an area of importance in this surgery. Blood already travels
relatively slowly in the veins. Now that we've removed an extra compartment in the heart, this problem is confounded.
So what if we tried to help this issue by changing the way we attach the SVC and the IVC to the pulmonary artery?
Normally, these vessels are attached head-on, meaning that they stay theoretically as a straight cylindrical tube.
Instead, let's connect them with a funnel-like extension, so that it opens up towards the pulmonary artery. This should theoretically do two things:
1. Decrease direct flow collision
2. Decrease downstream resistance
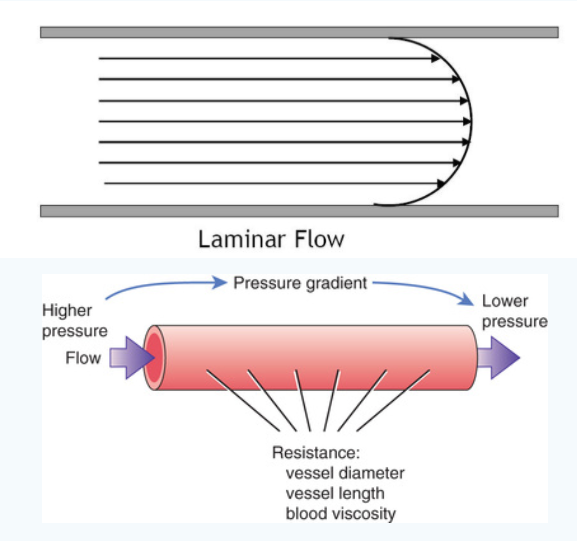
What does direct flow collision and downstream resistance mean?
Section the blood (or any fluid, really) in your mind as a bunch of lines moving together (see the picture on the left). These are
what are known as streamlines. In our cross-like model, we have blood moving down from the SVC and up from the IVC, eventually colliding
in the middle and flowing into the left and right pulmonary arteries. When they collide, they lose energy. Let's use this analogy: imagine
you're driving your car and a yield is coming up. Is it more energy-efficient for you to stop at the yield, then accelerate back to full speed?
Or is it better to "roll" into the yield and accelerate from a higher speed already? Definitely the second one. Now when we imagine those streamlines
in the SVC and IVC, they are directly colliding with each other and losing speed. If we introduce the funnel-like opening, they now have more room to
operate; more streamlines follow the edge of the vessel and don't collide head-on with other streamlines, meaning they lose less speed when they eventually
collide.
Downstream resistance is significantly easier to understand. Is it easier to push water through a tube that is five centimeters wide or ten centimeters wide?
Simply put, the bigger the radius of the tube, the easier time we'll have pushing that fluid through, assuming everything else stays the same.
Model and Results
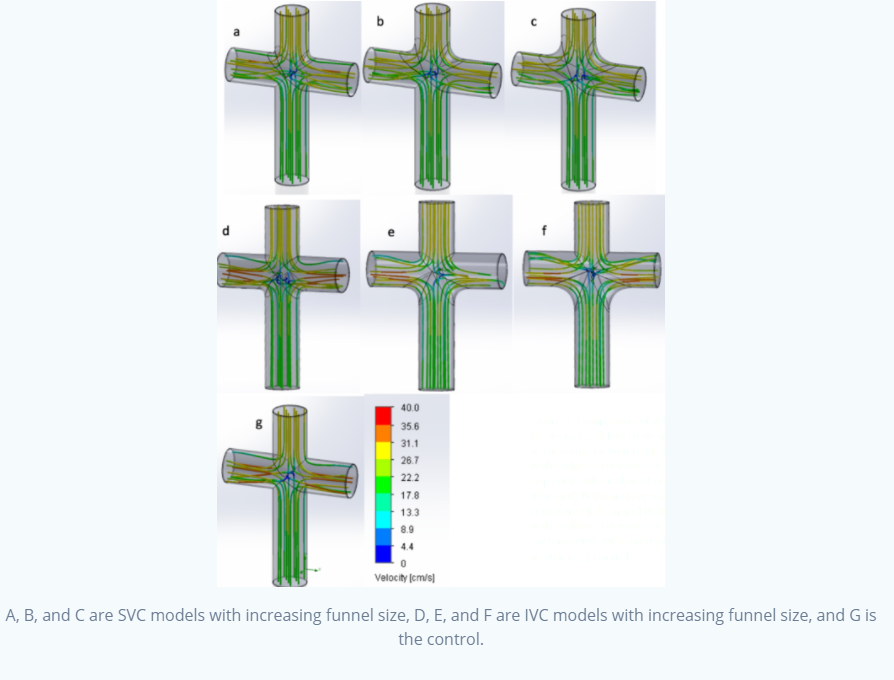
The models above were built using SOLIDWORKS, which were intended to mimic the TCPC connection we talked about earlier.
We had seven models: a control, and 10mm, 20mm, and 30mm funnels evaluated independently for the SVC and IVC.
What we found was that, with increasing radii of curvature (or in layman's terms, bigger funnels), downstream pressure was decreased.
What we also found was that the streamlines converged along the funnel, increasing velocity relative to other models when the streamlines
entered the left and right pulmonary artery. Take a look at the streamlines as they enter the collision area (this is more prominent in the
IVC models): you can see that there are less colliding streamlines.
Using fluid energy loss equations, we calculated the energy loss to be decreased by an average of 21% in the SVC models and by an average
of 38% in the IVC models, with as much as a 69% decrease in energy loss in the IVC models. This suggests there could be a huge potential
area of application where we could increase flow performance parameters especially in the IVC using this optimization.
Importance and Conclusion
This surgery is pretty much the only option for children that are born with this condition, outside of a full heart transplant.
So any optimization that can help improve post-surgical outcomes of these patients is important. While it may not entirely cure
the condition, it'll help them until we find something that does.
If you want something a little more technical/detailed, check out our final paper
for this project.
Thank you to my collaborators in this project: Lauren Loggi, Christopher Gearhart, and August Pfluger, as well as Dr. Nguyen at The College of New Jersey.